Consultation on Truvada will now be shelved, in move sexual health charities have described as ‘shameful’ and ‘failing those at risk’ of HIV
Charities and campaigners have reacted with anger and disbelief that plans to roll out a widely anticipated HIV prevention drug have been stalled by NHS England.
The sector had been waiting for the announcement of the first ever public consultation on the use of pre-exposure prophylaxis (PrEP) in the UK, now overdue by a month. Instead today NHS England announced it was not their responsibility to commission the drug.
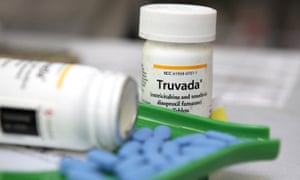
The HIV treatment pill Truvada containing PrEP can be taken on a daily basis – in a similar way that women take the contraceptive pill – by men who have sex with men to dramatically reduce the risk of HIV transmission. In February 2015, a Proud (pre-exposure option for reducing HIV in the UK: immediate or deferred) study reported that PrEP had effectively reduced the risk of HIV infection by 86%.
Terrence Higgins Trust, the largest provider of HIV and sexual health services in the voluntary sector, expressed shock and disbelief as structured plans for introducing the drug were shelved. Over 2,500 men who have sex with men are diagnosed with HIV each year in the UK, according to the trust.
Ian Green, chief executive officer of the charity said: “This figure has not changed in a decade. It is quite clear that although we have had some huge advances in HIV treatment, HIV prevention is something that we are still struggling with.”
Described as a “HIV gamechanger” and already available in the US, France, Canada, Israel, and Kenya, the process of public consultation would have been one of the final steps before a decision is made on NHS availability. The consultation response forms part of a submission to the clinical priorities advisory group, the body that it had been thought would make a decision on PrEP at its next meeting in June.
Instead, and despite its insistence that it holds no responsibility to commission HIV prevention services, NHS England said it would provide £2m over the next two years to run a number of early implementer test sites for 500 men “most at risk”.
Green said this will have very little impact compared to what had been planned. “By denying full availability of PrEP, we are failing those who are at risk of HIV. Today’s decision by NHS England to depart with due process, and, instead, offer a tokenistic nod to what has the potential to revolutionise HIV prevention in the UK, is shameful.”
Deborah Gold, chief executive of the National Aids Trust (NAT) agrees: “The decision is not informed by any due process; the amount of money is arbitrary; the claim that more ‘testing’ of PrEP is needed is disingenuous. 500 does not remotely cover the number of gay men at high risk of HIV nor meet the needs of heterosexuals at risk.”
Gold also said there was no clarity within the Department of Health, NHS or Public Health England as to who long-term is responsible to commission and fund PrEP and this was “simple maladministration” that would have “serious consequences”.
She said: “Over 5,000 gay men will get HIV over the next two years – very many of whom would not have done so if PrEP had been delivered as proposed.
“NAT share the anger and distress felt by many thousands of people across the country at NHS England’s decision to abandon its work to provide [the drug], near the very end of the process.”
Gay men, campaigners and healthcare professionals reacted with anger on Twitter after NHS England released their shock statement.









0 comments:
Post a Comment